REDUCE-IT (Vascepa) vs. STRENGTH (Epanova)
Why the difference in outcomes for these two high dose omega-3 trials?
Eric Roehm, MD, Oct. 2020, updated Dec. 2021
Why is mineral oil such a bad choice for placebo?
Detailed versions of the information on this website for the medical professional are the following:
1. Mineral oil, used in REDUCE-IT, is a flawed placebo and increases C-reactive protein levels.
2. Recent trials and Omega-3 nomenclature confusion (detailed) including: How Vascepa, Epadel, and Omacor omega-3 products all share one identical component.
3. Misleading Information in Amarin Corporation supported literature regarding omega-3 nomenclature.
4. Potential negative effects of Amarin employees doing all initial statistical analyses in the REDUCE-IT trial.
5. EVAPORATE (CT angiography-Vascepa) trial and mineral oil placebo.
REDUCE-IT and STRENGTH are the names of two large randomized trials using high dose omega-3s to assess effects on cardiovascular events such as heart attacks. REDUCE-IT1 used an omega-3 with the brand name Vascepa and STRENGTH2 used an omega-3 called Epanova. The following are two possible major reasons for the favorable outcomes in the REDUCE-IT omega-3 trial1, but not with the STRENGTH omega-3 trial3 :
1. The two trials studied different types of omega-3s. REDUCE-IT studied Vascepa which consists solely of EPA (icosapent ethyl1, also called eicosapentaenoic ethyl ester4) while STRENGTH studied Epanova which includes both EPA (eicosapentaenoic acid) and DHA (docosahexaenoic acid)2.
2. REDUCE-IT (Vascepa) used mineral oil as placebo1 which has adverse effects. Mineral oil increases inflammation within the body as evidenced by a marked increase in C-reactive protein. The use of mineral oil as a placebo is likely to have substantially worsened the placebo group outcomes and thereby made Vascepa outcomes look comparatively better.
Description of the trials: Both the REDUCE-IT and STRENGTH trials are large randomized trials studying the effects of omega-3 agents on outcomes like heart attacks and strokes. The entry criteria for the trials were quite similar which would lead to a similar study population. (Table 1- Comparison of Trials)
Treatment groups in both groups received omega-3 agents in high dose (4 grams/day). In REDUCE-IT, EPA (eicosapentaenoic acid) in the form of an ethyl ester was used1. In the STRENGTH trial, predominantly EPA and DHA (docosahexaenoic acid) were given in the free fatty acid form2. REDUCE-IT1 unfortunately used mineral oil as a placebo. STRENGTH2 used a corn oil placebo.
The positive results of the treatment group compared to the placebo group in REDUCE-IT were striking, with a statistically significant 31% in heart attacks and 28% reduction in total stroke rate1. (Total stroke rate reduction had never before been seen in a large randomized omega-3 trial.)
So why the dramatic difference in outcomes for the REDUCE-IT trial and the STRENGTH trial?
Possible contributing factors include:
1. Omega-3 agent:
-The REDUCE-IT trial used EPA alone (in an ethyl ester form).
-The STRENGTH trial included both EPA and DHA (in free fatty acid form)
2. Placebo:
–The REDUCE-IT trial used a mineral oil placebo which showed evidence of provoking an inflammatory response. (An inflammatory marker (high sensitivity C-reactive protein: hs-CRP) increased by 33% in the mineral oil placebo group1. An increasing hs-CRP has been associated with an increase in cardiac events such as heart attack5.
–STRENGTH trial used a corn oil placebo2.
3. Difference in patient populations between the trials did not appear to be the cause for the difference. The similar entry criteria for the trials led to very similar groups of trial participants. (The REDUCE-IT trial did have 71% of participants with prior CVD disease vs. the STRENGTH trial which had 56% with prior CVD disease. Trial results did not suggest this difference caused the disparity in outcomes.)
Why is mineral oil such a bad choice for a placebo?
Mineral oil causes inflammation. Inflammation is a process that increases the likelihood of heart attacks and strokes. Mineral oil increases C-reactive protein1,8, which is a marker of inflammation. An increased level of C-reactive protein has been associated with a marked increase in adverse cardiovascular events5. In REDUCE-IT, the mineral oil placebo group increased median levels by 33%1 compared to baseline, which is quite a significant increase.
No one can reliably say how much of the benefit attributed to Vascepa in REDUCE-IT was because of the omega-3 given vs. how much of the presumed beneficial effect was the result of mineral oil making the placebo group worse.
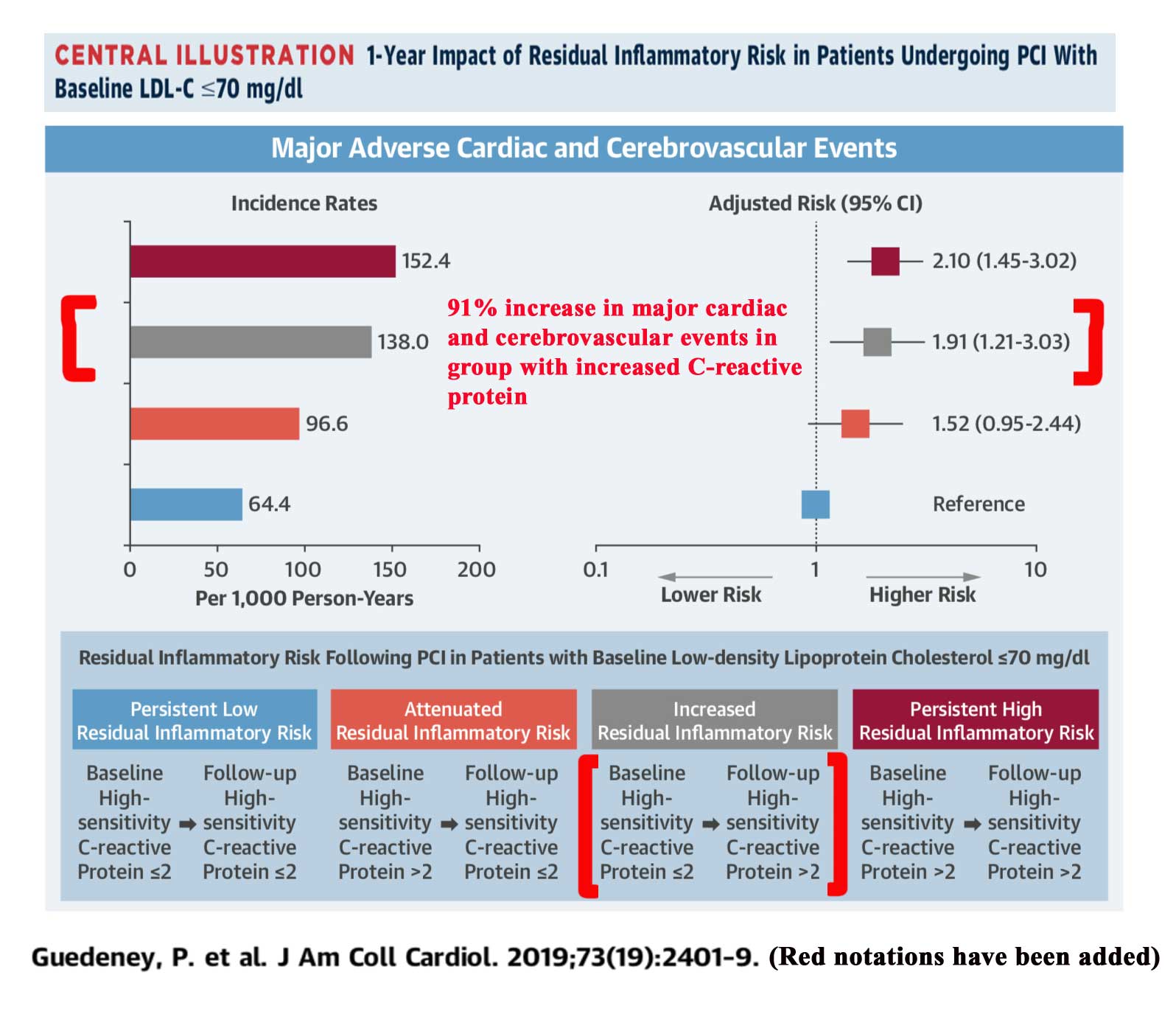
In a study of patients who had a heart artery procedure, when C-reactive protein (CRP) increased from less or equal to 2.0 mg/L, to greater than 2.0, the associated risk of cardiovascular events for the combined endpoint of heart attack, stroke, or death was increased by 91%5. This raises concern that in the REDUCE-IT trial, the use of mineral oil in the placebo group, may have led to more heart attacks and cardiovascular death than would otherwise be the case in the placebo group, causing the omega-3 group to appear better in comparison.
Of note, the study is observational, so the rise in CRP and increase in adverse cardiovascular events is an association and not cause and effect. Furthermore, the study examines a group who have all very recently undergone a cardiac procedure involving a heart artery, while only 70% of the participants in REDUCE-IT had cardiovascular disease.
Click on illustration below from study showing higher risk with increasing CRP:
Guedeney, P. et al.J Am Coll Cardiol 2019:73;2401-9 5
Comparison of REDUCE-IT and STRENGTH trials (Table 1):
| Trial | REDUCE-IT | STRENGTH |
| Published | 2019 | 2020 (Halted in Jan 2020 due to interim results indicating low likelihood of showing benefit with treatment.) |
| Participants | 8,179 | 13,086 |
| Duration (median) | 4.9 years | ~4 years (estimated) |
| Omega-3 agent | Vascepa: EPA in ethyl ester form.(called icosapent ethyl or EPA ethyl ester) 4ooo mg/day |
Epanova: free omega-3 fatty acids including both EPA & DHA (≥ 75%) 4ooo mg/day |
| Placebo | mineral oil 4000mg/day | corn oil ~4000mg/day |
| Participant Selection Criteria (very similar for trials) | Triglycerides 135-<500 mg/dL . Additional cardiovascular (CV) risk factors. or with preexisting CV disease (70% of participants had preexisting disease) ALL participants on statins |
Triglycerides 180-<500mg/dL AND HDL<42mg/dL male or <47 mg/dL for female. CV risk factors or with preexisting CV disease (≥50% of enrollees) ALL participants on statins |
| Placebo Group Outcomes (compared to baseline) |
Marked sustained elevation of an inflammatory marker (high-sensitivity C-reactive protein) while receiving mineral oil (median hs-CRP: 2.1mg/dL→ 2.8 mg/dL) |
|
| Treatment Group Outcomes (compared to placebo group) | Statistically significant: *31% reduction in myocardial infarction (MI) *28% reduction in total stroke rate that has never before seen in a large randomized omega-3 trial. (A positive effect of EPA or a negative effect of mineral oil?) *25% reduction in CV events (primary endpoint.) |
|
| Correlation of outcomes with baseline status | Benefit was present regardless of whether -preexisting CV disease present or triglycerides <200mg/dL or greater |
1. Bhatt DL, Steg PG, Miller M, et al. for the REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med 2019;380:11-22.
2. Rationale and design of the STRENGTH trial. Nicholls S, Lincoff AM, Bash D, et al. Clinical Cardiology 2018;41:1281-88.
3. Effect of high-dose omega-3 fatty acids vs corn oil on major adverse cardiovascular events in patients at high cardiovascular risk: the STRENGTH randomized clinical trial. Nicholls SJ, Lincoff AM, Garcia M, et al. JAMA. 2020;324(22):2268-2280.
(Above is the updated reference for STRENGTH trial. Reference used in Oct. 2020 when this page was initially written is the following:
STRENGTH trial announces early termination. Accessed Sept. 2020. https://www.astrazeneca.com/media-centre/press-releases/2020/update-on-phase-iii-strength-trial-for-epanova-in-mixed-dyslipidaemia-13012020.html)
4. Vascepa FDA Application- Pharmacology Review 2011, Accessed May 2020.
https://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/202057Orig1s000PharmR.pdf
5. Guedeney P, Claessen BE, Kalkman DN, et al. Residual Inflammatory Risk in Patients With Low LDL Cholesterol Levels Undergoing Percutaneous Coronary Intervention. J Am Coll Cardiol. 2019;73:2401-09.
6. Epanova prescribing information. Accessed Sept. 2020.
https://www.accessdata.fda.gov/drugsatfda_docs/label/2004/21654lbl.pdf
7. Benes LB, Nikhil S Bassi NS, and Davidson. .MH. Omega-3 carboxylic acids monotherapy and combination with statins in the management of dyslipidemia. Vasc Health Risk Manag. 2016;12:481–490.
8. Ballantyne CM, Bays HE, Kastelein JJ, et al. Efficacy and safety of eicosapentaenoic acid ethyl ester (AMR101) therapy in statin-treated patients with persistent high triglycerides (from the ANCHOR study). Am J Cardiol 2012;110:984-92.