Mineral oil is pro-inflammatory and causes changes associated with adverse cardiovascular events
Eric Roehm, MD, Oct. 2020
No one can reliably say how much of the reduction in cardiovascular events seen in the REDUCE-IT trial1 is from the positive effects of the omega-3 intervention (eicosapentaenoic acid ethyl ester) vs. how much is from the negative effects of mineral oil on the placebo group’s inflammatory status.
REDUCE-IT (Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia), a randomized trial of 8,179 patients, used mineral oil as the placebo.1 REDUCE-IT compared Vascepa (icosapent ethyl*) to mineral oil. The mineral oil group received a total of 4 one-gram capsules divided into two daily doses and given with food for a median of 4.9 years.1 (icosapent ethyl and eicosapentaenoic acid ethyl ester are synonyms for the identical chemical structure)
Mineral oil is not a neutral placebo:
Mineral oil is used as a laxative because it decreases water absorption from the gut. In a study of children receiving mineral oil for up to 4 months, beta-carotene levels decreased, but there was no adverse effect on retinol or alpha-tocopherol levels.2 The only large randomized human trials of mineral oil are those involving Vascepa.1,3 Mineral oil, unfortunately, is pro-inflammatory, as evidenced by increasing high sensitivity C-reactive protein (hs-CRP) in the mineral oil treated group.1,3 (LDL cholesterol increased in the mineral oil treated group as well, but by a much lesser extent.1)
In the REDUCE-IT trial, the placebo mineral oil group showed evidence of a significant inflammatory response. There was a substantial and statistically significant 33% increase in high sensitivity C-reactive protein (hs-CRP) levels in the mineral oil “placebo” group (2.1→2.8 mg/L; baseline levels before and after 2 years of mineral oil, p<0.001).1 (The elevated hs-CRP levels persisted through the last visit of the trial which lasted a median duration of 4.9 years.1)
Increasing hs-CRP levels and increased adverse cardiovascular outcomes:
Increasing high sensitivity CRP levels (hs-CRP) have been associated with a striking increase in total major adverse cardiovascular events (myocardial infarction, stroke, or death). An observational study of 3,013 patients following percutaneous coronary intervention (PCI), who had serial C-reactive protein levels available at baseline and at least 4 weeks in follow-up, was published in 2019.4 Those patients with an increase in C-reactive protein going from less than or equal to 2 mg/L to greater than 2 mg/L had a statistically significant 91% increase in risk of the the combined endpoint (MI, stroke, or death) at one year follow-up.4 (There was a statistically significant 79% increase in the risk of myocardial infarction.)4
Residual Inflammatory Risk in Patients with Low LDL Cholesterol Levels Undergoing Percutaneous Coronary Intervention. Guedeney, P, et al, J Am Coll Cardiology. 2019;73(19):2401-9 4
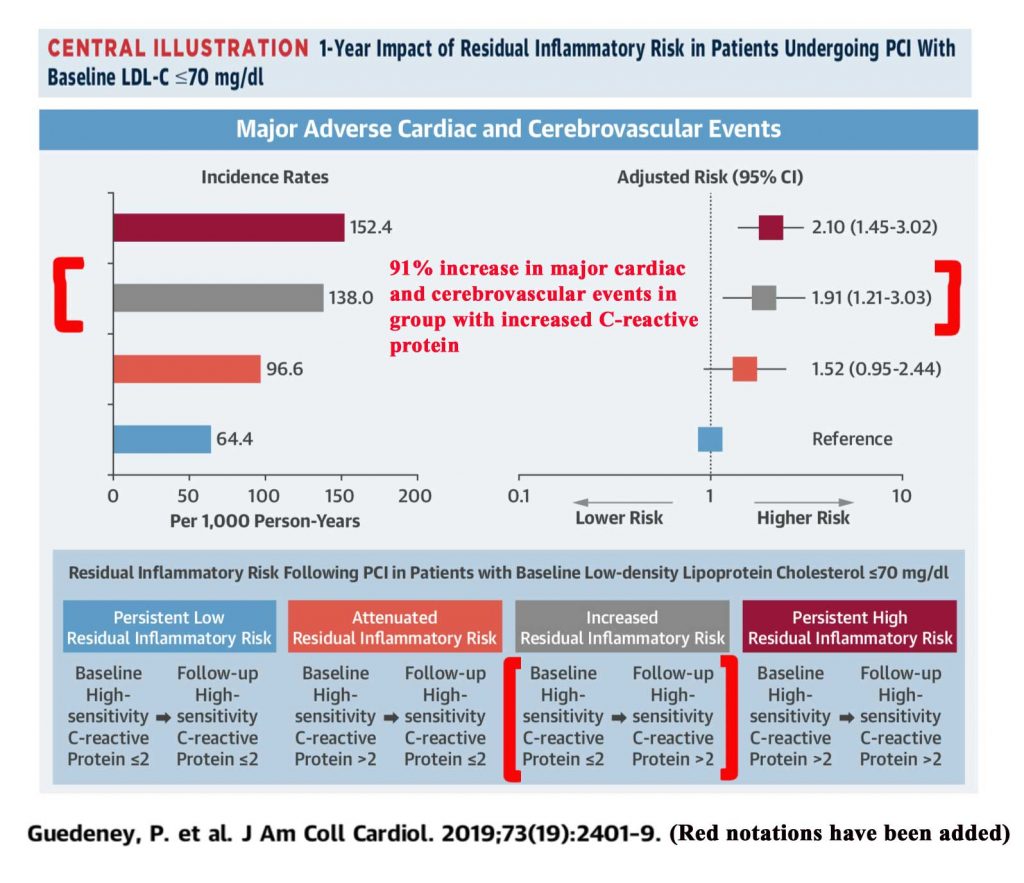
Illustration is from Guedeney et al. article4 (additional notations made in red)
Since the above study of 3,013 patients was not a randomized trial, the increase in C-reactive protein can only be said to be associated with the worse outcomes, not the cause of them. Also the participants in this study were different from REDUCE-IT, in that they all underwent a percutaneous coronary intervention (PCI). In REDUCE-IT, only 70% of participants had preexisting cardiovascular disease.1
Comparison to the CANTOS trial5:
Another way of looking at this risk would be to extrapolate from the CANTOS trial (Canakinumab Anti-Inflammatory Thrombosis Outcomes Study)5. However, assumptions would have to be made that would make this comparison not reliable. In CANTOS, patients randomized to canakinumab had decreased hs-CRP levels and had a significantly lower rate of cardiovascular (CV) events. If one assumes that relation of the increase of hs-CRP levels and CV events in REDUCE-IT is linearly and inversely proportional to the decrease of hs-CRP levels and CV events in CANTOS, then 0.05 of the 0.25 decrease in the hazard ratio for the primary endpoint of REDUCE-IT (i.e., 20 % of the total benefit) is attributable to mineral oil.
However, the assumption that there is a linear inverse relation to the CANTOS trial data is not necessarily correct. It is possible that increasing levels of hs-CRP levels may at times correspond to the development of a threshold level of inflammation that increases CV events in a non-linear manner.
No one can reliably say how much of the reduction of CV events in REDUCE-IT is from Vascepa and how much is from the negative effects of the mineral oil.
References:
1. Bhatt DL, Steg PG, Miller M, et al. for the REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med 2019;380:11-22.
2. Clark JH, Russell GJ, Fitzgerald JF, et al. Serum beta-carotene, retinol, and alpha-tocopherol levels during mineral oil therapy for constipation. Am J Dis Child 1987;141:1210-2.
3. Ballantyne CM, Bays HE, Kastelein JJ, et al. Efficacy and safety of eicosapentaenoic acid ethyl ester (AMR101) therapy in statin-treated patients with persistent high triglycerides (from the ANCHOR study). Am J Cardiol 2012;110:984-92.
4. Guedeney P, Claessen BE, Kalkman DN, et al. Residual Inflammatory Risk in Patients With Low LDL Cholesterol Levels Undergoing Percutaneous Coronary Intervention. J Am Coll Cardiol. 2019;73:2401-09.
5. Ridker PM, Everett BM, Thuren T, et al. for the CANTOS Trial Group. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease.
N Engl J Med 2017, 377:1119-1131
